By Gabriel Martínez del Mármol
Updated: 05/04/2020
INTRODUCTION
Snakes (Serpentes Linnaeus, 1758) are within the order Squamata, of the class Reptilia, within the phylum Chordata (Pyron et al., 2013).
They inhabit the majority of the biomes of planet Earth and within this large suborder, the vipers are probably one of the best known and recognized of all the snakes.
The subfamily, Crotalinae Oppel, 1811 has a wide global distribution and thus a greater number of genera and species within that subfamily (Wallach et al., 2014). That family, known as the pit vipers, occurs in the Americas and throughout Asia from the islands of southeast Asia ( Philippines-Indonesia), in the east to Azerbaijan in the west (McDiarmid et al., 1999; Gloyd & Conant 1990). In this subfamily we find genera with extreme phenotypic variability such as the rattlesnakes of the genus Crotalus Linnaeus 1758. Also included in this subfamily are the largest vipers on Earth, those in the genus Lachesis Daudin, 1803, and the great variability of arboreal vipers which are found within the genera Bothriechis Peters 1859, Trimeresurus Lacépède 1804 and Tropidolaemus Wagler 1830. Several new genera have recently been described (Cryptelytrops, Himalayophis, Parias, Peltopelor, Popeia and Viridovipera) Malhotra and Thorpe 2004. A number of species are quite adept at camouflage such as those in the genus Agkistrodon Palisot de Beauvois 1799 and Protobothrops Hoge & Romano-Hoge 1983. Some vipers, such as those in the genus Gloydius Hoge & Romano-Hoge 1981 can be found at high altitude, even reaching elevations up to 4.880m (16.000 feet)(Mc Diarmid et al., 1999; Shi et al., 2017).
The true, or Old World vipers are where we can find the family with the most extreme phenotypic adaptations. Apart of the spider-tailed viper, Pseudocerastes urarachnoides Bostanchi, Anderson & Papenfuss 2006, which has the most advanced lure mechanism in all the animal kingdom, are snakes in the genus, Bitis Gray 1842, and it is here where we find the most diverse phenotypic variability of all the vipers, and likely in all snakes species.
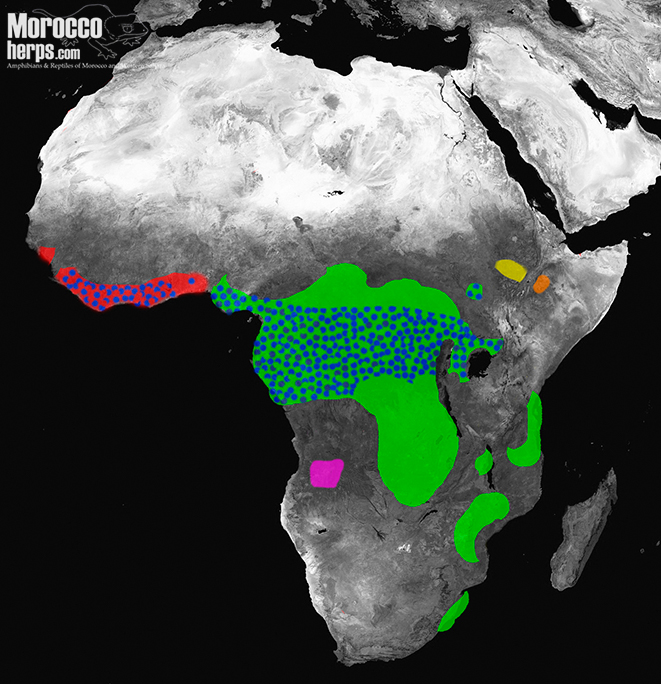
Whereas, for example, the genus Daboia Gray 1842 has the greatest geographic distribution, extending from the Atlantic Coast of Morocco in the west to the islands of Indonesia in the east (Das, 2012), the genus Bitis is primarily one from Africa, and more exactly specifically, of sub-Saharan Africa (Phelps 2010). In fact, although there are some small populations in Arabia and North Africa, it is in sub-Saharan Africa where the genus reaches its most diverse and varied evolutionary traits.
THE BITIS SPECIES
That extreme phenotypic variability is also reflected in the genetic data as well. Lenk et al. (1999) used molecular data (immunological distances and mitochondrial DNA sequences) to estimate the phylogenetic relationships among the various species of Bitis, and identified four major monophyletic groups for which they created four subgenera. Further analyses have confirmed that genetic diversification in the genus Bitis (Barlow et al., 2013; Pyron et al., 2013; Barlow et al., 2019, Fig 1).
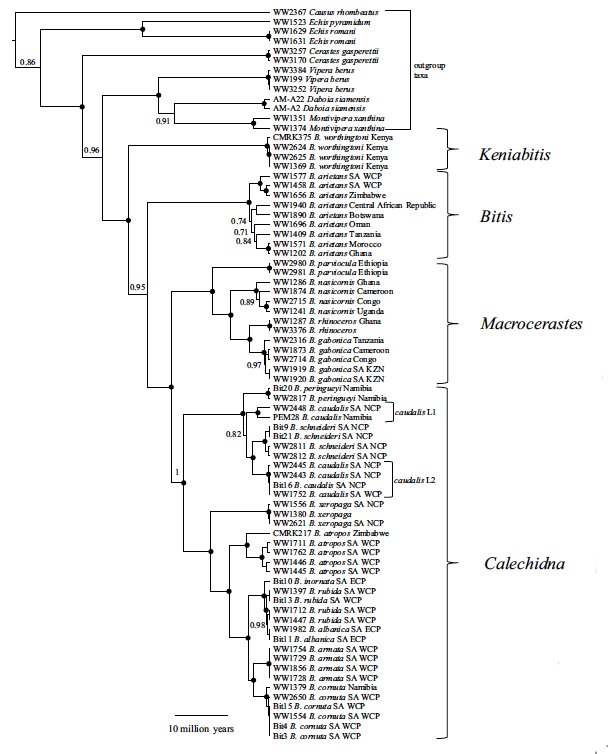
1) BITIS
The subgenus Bitis is composed of a unique species, Bitis arietans (Merrem, 1820). Bitis arietans is commonly known as a puff adder, because when it feels threatened, it will hiss very loudly as a warning usually before striking.
Adults average less than one meter in total length, although some individuals can reach lengths of more than 120cm (Marlow et al., 2003). However, as a species they are very stout and bulky snakes so even though they are not very large, they are nonetheless quite impressive-looking snakes.

Like most of the Bitis species, they usually move slowly and with a rectilinear locomotion, although if they feel threatened, they can move with rapid sidewinding movements and can disappear quite quickly especially if they find a place of refuge.
In South Africa, coloration and pattern can vary dramatically even in individuals within a single population, whereas in Morocco, for example, the variability of patterns and coloration is very consistent (Martínez del Mármol et al., 2019). Generally, body coloration and pattern are very similar to the habitat where the snake lives, helping it to blend in with its surroundings.
Like most vipers, the genus Bitis, specializes in hunting by ambushing its prey (Marais 2004; Miller et al., 2015).
In most regions of Africa, Bitis arietans occur in grassland and savannah, whereas in Arabia (at least in Oman) they are restricted to the surroundings of the rivers in the areas influenced by the seasonal monsoons (Arnold 1980; Carranza et al., 2018). In Morocco, they also are more limited in occurrence due to the weather than to the habitat. In the coastal area from the Souss Valley to Dahkla they can be found in a variety of places, such as those surrounding rivers and rocky areas but their preferred habitat are the large steppes with sandy soil and abundance of mammal burrows.

In Morocco, Puff Adders are one of the few snakes that can be found active all year long. Records of observations in February through May and again in August throught December indicate that their activity period is different to those of other Mediterranean-type snakes, and seems to depend more on the rains to be active than the season of the year. A similar situation also occurs in Morocco with the colubrid snake, Dasypeltis sahelensis Trape & Mané 2006 (Jiménez Robles et al., 2017)
Although it seems to be a relict species (Bons & Geniez 1996), Bitis arietans is very abundant in its range in Morocco, probably because in Morocco this species gives birth to large numbers of newborns. In general, this species can average litters of over 50 young which is rather typical; and a female, originally from Kenya and maintained in a Czech zoo gave birth to 156 young, the largest known litter for any species of snake (Mehrtens 1987, Spawls et al., 2004).
Two subspecies are currently recognized: Bitis arietans somalica, Parker 1949 for some populations in Somalia and northern Kenya (fig 4.6), and the nominate subspecies for the remaining populations (fig 4 not including 4.6). That does not seem to reflect the correct diversity in the species, where there are at least three big clades according to the genetic analysis: 1) Morocco, Arabia, West Africa and part of East Africa; 2) Central Africa and other parts of East Africa; and 3) Southern Africa populations where also a high diversity has been reported (Barlow et al., 2013). It seems that a revision of this species might be necessary, one that would be of great importance, both biologically and medically for Africa.

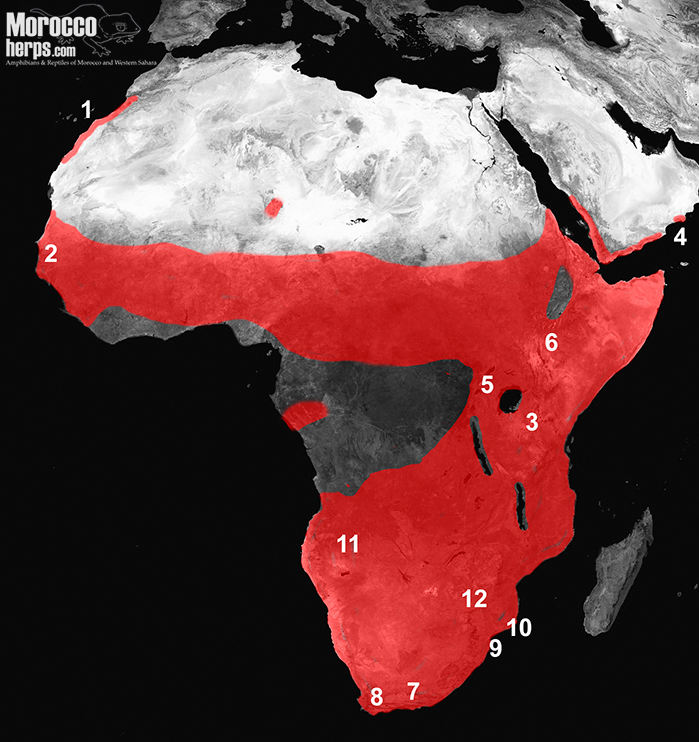
Its excellent camouflage, high reproductive capacity, potent venom and rapid strike make it one of the most successful predators in Africa, although unfortunately, they are also the responsible for numerous snakebites fatalities in Africa (Mallow et al., 2004).
2) CALECHIDNA
This subgenus consists of the small-adders / dwarf adders that can be grouped in 2 big clades (Barlow et al., 2019):
- The arenicolous clade (“sandy” adders): Bitis caudalis species complex (A.Smith 1839), Bitis schneideri (Boettger 1886), Bitis peringueyi (Boulenger 1888)
- The rupicolous clade (“rocky” adders): Bitis xeropaga Haacke 1975, Bitis atropos (Linnaeus 1758), Bitis cornuta (Daudin, 1803), Bitis inornata (Smith, 1838), Bitis rubida Branch 1997, Bitis albanica Hewitt 1937 and Bitis armata (Smith 1826)
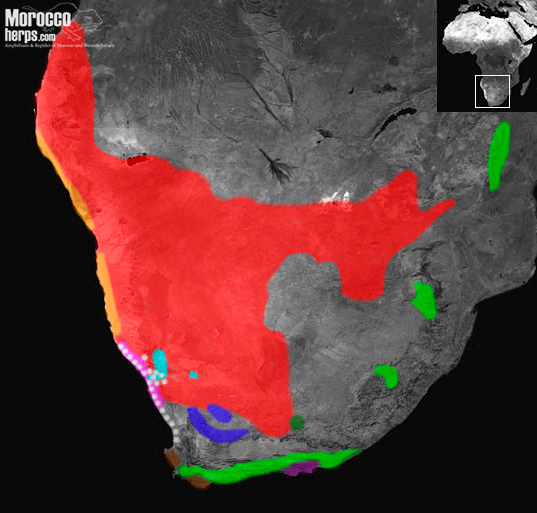

THE SANDY ADDERS
In the Saharan Desert and the Middle East, the vipers specialized in living in sandy habitats are members of the genus Cerastes Laurenti 1768. In the Balochistan desert the vipers specialized living in sandy environs belong to the genus Eristicophis Alcock and Finn, 1897. Within the pitvipers, Crotalus cerastes, Hallowell 1854, inhabits the deserts of some regions of the southwestern United States and Mexico. In the Namib Desert, of southwestern Africa, considered the oldest desert on Earth, vipers of the genus Bitis are the ones that have most successfully colonized the region.

In southern Morocco, Cerastes vipera (Linneaus 1758) is the viper species that predominately inhabits large sand dunes. These snakes have eyes that are almost on the top of their heads, an indication that they are ambush specialists that wait for their prey remaining completely hidden and buried under the sand having only their eyes exposed. This behavior is nearly identical to that of Bitis peringueyi. Both species are of similar size, have their eyes in similar locations, and share similar hunting strategies. Both species prefer the boundaries of large dunes, where there are numerous small bushes in which they can hide. A similar situation occurs in the family Boidae, Gray 1825, in the species Eryx jayakari Boulenger 1888, where the snakes share an adaptation to living in dunes.

In southern Morocco there is another species of viper that inhabits the large dune regions: Cerastes cerastes (Linneaus, 1758). Whereas Cerastes vipera seems to be more restricted to the sandy areas (with a few exceptions, Bouazza et al. 2020), the other viper species also inhabits rocky habitat. Cerastes cerastes is a larger species, and most populations have scales that resemble horns above each eye, and some specimens can have extremely variable coloration patterns. A similar situation occurs in Southern Africa with Bitis caudalis. They have been found together with Bitis peringueyi in many places although they are not quite as specialized in living in sand dunes, and can be found in large rocky steppes and intermediate habitats.

Genetic analysis has shown at least 2 different genetic lineages within Bitis caudalis, one close related to Bitis schneideri, a species considered as the smallest viper in the world with an average size of 20cm and maximum size of 28cm (Barlow et al. 2019; Marais 2020).


ROCK DWELLING ADDERS
Bitis xeropaga, Bitis atropos, Bitis cornuta, Bitis inornata, Bitis rubida, Bitis albanica and Bitis armata are all small to medium species of vipers that rarely exceed more than 50cm in length and inhabit mainly rocky habitats and grasslands in the mountains or coastal areas in Southern Africa.
One of the most unique species of vipers is Bitis cornuta. While there are a number of vipers genera with horns on their heads: e.g. Crotalus Linneaeus 1758, Cerastes Laurenti 1768, Ophryacus Cope 1887 and Bothriechis Peters 1859, none of them possess horns on their heads similar to those of Bitis cornuta.

The species Bitis atropos can live in biomes up to 3.000m (9.850 feet) in elevation, in a mountain habitat where in other parts of the world, for instance, Europe, Middle East, or North Africa, one would expect to find individuals of the genera, Vipera Laurenti 1768 or Montivipera Nilson, Tuniyev, Andren, Orlov, Joger & Herrmann 1999. They are extremely variable in pattern and color, and can live in mountain habitats as well as in coastal areas. Fitzimons (1959) described the subspecies B. atropos unicolor mainly to be “almost uniformly khaki to reddish brown above and apparently a smaller snake than typical atropos”.

Bitis rubida is an endemic species of several mountains of South Africa (Cape Fold Mountains from the Cederberg in the north into the Swartberg and Langberg and Baviaans mountains) and it is paraphyletic with respect to B. albanica in the mitochondrial gene tree. Although it is commonly known as the “red adder”, that morphotype seems to be restricted to the Cederberg (Marais 2020) whereas the common and more widespread pattern seems to be the contrasted grey-dark one.

Bitis xeropaga can be only found in some rocky mountains of the Orange River valley into the Fish River Valley and in southern Namibia, and its color varies greatly depending on the coloration of the rocks in its habitat.

Concluding with this subgenus, are some dwarf adders species of the “rupicolous clade”: Bitis inornata, Bitis albanica and Bitis armata have a very small geographic range and are limited to only a few narrow mountain or coastal habitats (Phelps 2010).



3) MACROCERASTES
This is the subgenus of the forest vipers. There is a large clade within the species Bitis gabonica (Duméril, Bibron & Duméril 1854) and Bitis rhinoceros (Schlegel 1855), often called Gaboon vipers of West and East Africa. Bitis nasicornis (Shaw 1792) are referred to as Rhinoceros Vipers and the endemic Bitis parviocula, of Ethiopia, is called the Bale Mountain Adder, Bohme 1977.
Macrocerastes species are mainly forest vipers, they are typical snakes of the rainforest and agricultural areas in the edges of these forests.

Bitis gabonica is well-known as the species with the longest fangs of any snake species on Earth, some reaching lengths of up to 5cm (2 inches). Although this species can be found in agriculture fields habitats, they are typical species of primary and secondary forests sporting a dorsal pattern that blends remarkably well with the forest floor of dead leaves.


Bitis rhinoceros was previously considered a subspecies of Bitis gabonica, with similar aspects of behavior and habitat preference. Both are some of the most impressive snakes in the world and despite their large size (they can reach lengths up to 175cm; Spawls et al. 2004) they often remain completely unnoticed in their native habitat.
Bitis nasicornis is considered one of the jewels of African wildlife. They usually show amazing color patterns and have two large, pronounced scales on the snout. Although they have a somewhat slow and awkward method of locomotion in the way they move, which is similar to other species of large vipers in this genus, some records of this species are of individuals found in branches of large trees 2-3 meters above the ground. This arboreal behavior is very rare in Old World vipers, except in the genus Atheris Cope 1862, and very exceptionally in other genera (Macrovipera, Nilson et al., 1999; Vipera, Aleksandar Simovic, pers.comm.) and is unique within the genus Bitis, where arboreal behavior is not typical (Marlow et al., 2003).

Bitis parviocula, which is different than the rest of species in the subgenus, has no head ornamentations, but usually has a vivid bright yellowish color.

Bitis harenna Gower, Wade, Spawls, Bohme, Buechley, Sykes & Colston 2016 was the most recent species described in the genus, and was considered to be part of a population of Bitis parviocula (Wallach et al. 2014), so it will likely belong to this large clade.

The species, Bitis heraldica (Bocage 1889) is one of the least known vipers in Africa. Although it was considered in the past a synonym of Bitis peringueyi by Boulenger (Gonçalves et al. 2019), it is included here in the subgenus Macrocerastes due to its habitat selection, which is not the rocky or sandy habitat typical of the subgenus Calechidna, and due to Its distribution range, which extends far into of South Africa seems to be the origin of the subgenus Calechidna (see Fig. 6). Recent genetic analysis place B. heraldica among other members of the subgenus Macrocerastes (Ceríaco et al. 2019).

4) KENIABITIS
This subgenus only comprises the specie, Bitis worthingtoni Parker 1932, a medium sized viper with a maximum length of 50cm, endemic to the Rift Valley of Kenya.
After the Bitis arietans subgenus Bitis, Keniabitis is the most distant genetic subgenus within the remainder of the Bitis species (Barlow et al. 2019).
Like Bitis caudalis, this species has also horns above the eyes, and characteristically a pattern of two dorsal longitudinal lines on both sides of the body, from the neck to the tail.


THE FUNCTION OF THE HORNS
In general, the existence of horns in snakes elicits various debates. In the species within the genus Bitis, horns above the eyes or the snout are present, although a clear cause for this is still not completely known.
Klauber (1956) mentioned that the horns in Crotalus cerastes serve as radiators of heat or as shade covers for the eyes. Cowles (1953) regarded them simply as a whim of evolution. Cohen & Myres (1970) suggest that they have the function of an eyelid protecting the snake’s eyes while crawling through burrows. Wagner and Wilms (2010) suggested that perhaps horns could have a sexual function as in other reptile groups (e.g. Chamaeleonidae Gray 1825, Agamidae Spix 1825).
Most of these theories are not very consistent. In the case of horn/horns on the snout, some snakes, such as Ahaetulla pulvurulenta (Duméril, Bibron & Bibron 1854), Gonyosoma boulengeri Mocquard 1897 or Langaha madagascariensis Bonnaerre 1790, it is postulated that the main function of these horns could be to aid in the snakes camouflage in branches (with also a possible sexual function in Langaha due to the different shapes of the horn in males and females; Glaw and Vences 2007), and in other cases, such as in Erpeton tentaculatum Lacépède 1800, the horns or tentacles have a sensorial function to aid the snakes in catching fish (Catania et al., 2010). In vipers it is clear that it cannot be a whim of evolution because we can find such examples in many genera in different regions of the world. We find a clear example in two European vipers: Vipera latastei Bosca 1878 and Vipera ammodytes Linnaeus 1758. The horn needs to have a viable reason for its development considering that two different vipers has evolved horns although one is formed by a few elongated scales and the other horn is composed by many granular scales.

The theory that horns are needed to protect a snake eyes while in burrows is also not completely valid, because most snakes species that spend time underground in mammal burrows and narrow cracks do not have horns on their snouts or above their eyes. Even within Bitis, there are horned and hornless species occupying both desert or mountains habitats, as in Cerastes sp. for example, and there are numerous populations of hornless snakes, such as in the subspecies Cerastes cerastes mutila described by Sochurek (1979) and Cerastes gasperetti mendelssohni Werner & Sivan in Werner et al 1999.
Although horns may have a sexual function, since in some species both sexes have horns perhaps the reason is not for a male’s competition, but possibly a way vipers may utilize horns for sexual stimulation.
Another hypothesis might be that the horns look like seedling vegetation or newly emerging sprouts which may attract rodents to them (like a type of lure), this may also explain why Bitis peringueyi or Cerastes vipera do not possess horns, Bitis peringueyi or Cerastes feed primarily on various lizards, whereas Bitis caudalis, Cerastes cerastes and Crotalus cerastes can prey on small mammals (Shine et al., 1998; Mallow et al., 2003; Webber et al., 2016). These three, horned snakes species are usually partially buried so their rodent prey would only see two small plant-like structures before being preyed-upon. However, this hypothesis seems doubtful considering that we see horns above the eyes in many arboreal snakes that seldom or only rarely feed on rodents (for example Atheris Cope 1862 or Bothriechis Peters 1859).
The probable evolutionary reason for the presence of horns could be just for the purpose of camouflage within the snake´s habitat. In the example where three species of vipers from different regions of the world live in similar habitats (Bitis caudalis, Cerastes cerastes and Crotalus cerastes) that have horns, indicates that there is likely an important reason for possessing horns and is an example of convergent evolution. Furthermore, looking in detail within the genus Pseudocerastes, it is apparent that their horns are composed of granular scales (similar to those of V. ammodytes on the snout), which further supports the importance of horns in these species. And, although the false horned vipers can live in sandy areas, they are more typically a rock-dwelling species that ambush their prey or position themselves inside rock crevices without burying themselves.
Concluding with this part, it will be mentioned the hypothesis of horns for defense. Juan Timms commented to the author that according to his experience both in field and with captive vipers, the function of the supraocular horns should be to protect the eyes when they capture a prey. Rodents, lizards or birds have the ability to scratch with their feet and cut in the snake’s eyes. In fact, all supraocular horned vipers, arboreal or terrestrial, tend to hold prey when they hunt it, not like other vipers that bite and release, and then eat the dead prey.


CONCLUSIONS
There are many genera of snakes in the world, some with extreme adaptations to nearly all the various habitats. The genus Bitis has a restricted distribution primarily in Africa, but their species have evolved to fit into all the African habitats: the coastal areas, deserts, rain forests, savannahs, grasslands and high mountainous regions. The phenotypic variability of these vipers varies from the smallest species on earth to those with the largest fangs. The overall coloration of the various species range from typical, sandy-brown to very dark backgrounds and can include shades of yellow, red, green and even blue. Some species of Bitis even have the most spectacular head ornamentations among all the living snakes, and despite the many hypothesis, there are no conclusive theories about the function of these horns on the snout or above the eyes.
The presence of the genus Bitis in Morocco is proof of the ecological plasticity of this genus to different environments. Bitis are extremely adaptive animals that can be common, despite the fact that the presence of many other viper species or predators may occur in the same region.
AKNOWLEDGEMENTS
To Paul Freed, Fernando Martínez-Freiría and Dylan Leonard for their assistance with photos and in the editing of this article. To Keir and Alouise Lynch, Jonas Arvidsson, Juan Timms, Luis Ceríaco, Dayne Braine, Jurgen Gebhart, Jo Balmer, Alex Rebelo, Tomas Mazuch, Alberto Sánchez Vialas, Felix Hulbert and Krystal Tolley for their assistance with photos or permission for the use of various documents in this article.
BIBLIOGRAPHY
- Arnold EN. 1980. The scientific results of the Oman flora and fauna survey 1977 (Dhofar). The reptiles and amphibians of Dhofar, southern Arabia. Journal of Oman Studies Special Report (No. 2): 273-332
- Barlow A, Baker K, Hendry CR, Peppin L, Phelps T, Tolley KA, Wüster CE, Wüster W. 2013. Phylogeography of the widespread African puff adder (Bitis arietans) reveals multiple Pleistocene refugia in southern Africa. Molecular Ecology, 22, 1134–1157
- Barlow A, Wüster W, Kelly CMR, Branch WR, Phelps T, Tolley KA. 2019. Ancient habitat shifts and organismal diversification are decoupled in the African viper genus Bitis (Serpentes: Viperidae). J Biogeogr. 00:1–15
- Bouazza A, Laïdi K, Martín J. 2020. New record of the Sahara sand viper, Cerastes vipera (Linnaeus, 1758), from north-eastern Morocco. Herpetology Notes 13: 203-205
- Carranza S, Xipell M, Tarroso P, Gardner A, Arnold EN, Robinson MD, et al. 2018. Diversity, distribution and conservation of the terrestrial reptiles of Oman (Sauropsida, Squamata). PLoS One 13 (2): e0190389
- Catania KC, Leitch DB, Gauthier D. 2010. «Function of the appendages in tentacled snakes (Erpeton tentaculatus)». Journal of Experimental Biology 213: 359-367
- Ceríaco L, Tolley K, Marques M, Heinicke M, Blackburn D, Bauer A. 2019. A Dwarf among giants: Biogeography and Phylogenetic Position of the Elusive Angolan Adder, Bitis heraldica (Bocage, 1889). African Herp News 72: 51-52.
- Cohen AC, Myres BC. 1970. A function of the horn in the sidewinder rattlesnake Crotalus cerastes, with comments on other horned snakes. Copeia 3, 574-5.
- Das I. 2012. A Naturalist’s Guide to the Snakes of South-East Asia: Malaysia, Singapore, Thailand, Myanmar, Borneo, Sumatra, Java and Bali. John Beaufoy. 160 pp.
- Dobiey M, Vogel G. 2007. Venomous Snakes of Africa. Giftschlangen Afrikas. Edition Chimaira, Frankfurt.
- Fitzsimons V. 1959. Some new reptiles from southern Africa and southern Angola. Annals Transvaal Mus. 23:405-409
- Glaw F, Vences M. 2007. A Field Guide to Amphibians and Reptiles of Madagascar 3rd edition. Köln: M. Vences & F. Glaw Verlags GbR
- Gloyd HK, Conant R. 1990. Snakes of the Agkistrodon Complex: A Monographic Review. Society for the Study of Amphibians and Reptiles. 614 pp.
- Gonçalves FMP, Braine D, Bauer AM, Valério HM, Marques MP, Ceríaco LMP. 2019. Rediscovery of the poorly known Angola Adder, Bitis heraldica (Bocage, 1889) (Serpentes: Viperidae): new records, live photographs, and first case history of envenomation Herpetological Review 50 (2): 241-246
- Gower DJ, Wade EOZ, Spawls S, Böhme W, Buechley ER, Sykes D, Colston TJ. 2016. A new large species of Bitis Gray, 1842 (Serpentes: Viperidae) from the Bale Mountains of Ethiopia Zootaxa 4093 (1): 041–063
- Jiménez-Robles O, León R, Soto Cárdenas M, Rebollo B, Martínez G. 2017. Contributions to the natural history and distribution of Dasypeltis sahelensis Trape & Mané, 2006, in Morocco. Herpetozoa 30 (1/2), 80–86
- Klauber LM. 1956. Their habits, life histories, and influence on mankind. Univ. California Press
- Lenk P, Herrmann H-W, Joger U, Wink M. 1999. “Phylogeny and Taxonomic Subdivision of Bitis (Reptilia: Viperidae) based on molecular evidence”. Kaupia. 8: 31–38.
- Malhotra A, Thorpe RS. 2004. A phylogeny of four mitochondrial gene regions suggests a revised taxonomy for Asian pitvipers (Trimeresurus and Ovophis). Molecular Phylogenetics and Evolution 32, 83-100.
- Mallow D, Ludwig D, Nilson G. 2003. True Vipers: Natural History and Toxinology of Old World Vipers. Krieger Publishing Company, Malabar, Florida. 359 pp
- Mallow D, Ludwig D, Nilson G. 2004. True Vipers: Natural History and Toxinology of Old World Vipers. Malabar, FL: Krieger Publishing Company.
- Marais J. 2004. A Complete Guide to the Snakes of Southern Africa, 2nd ed. Struik Publishers, 312 pp.
- Marais J. 2020. Dwarf Adders of Southern Africa. africansnakebiteinsitute.com. Consulted the 17 of March of 2020
- Martínez del Mármol G, Harris DJ, Geniez P, de Pous P, Salvi D. 2019. Amphibians and Reptiles of Morocco. Frankfurt, Germany, Edition Chimaira. 478 pp.
- McDiarmid RW, Campbell JA, Touré T. 1999. Snake Species of the World: A Taxonomic and Geographic Reference, vol. 1. Herpetologists’ League. 511 pp.
- Mehrtens JM. 1987. Living Snakes of the World in Color. New York: Sterling Publishers. 480 pp.
- Miller AK, Maritz B., McKay S, Glaudas X, Alexander GJ. 2015. An ambusher´s arsenal: chemical crypsis in the puff adder (Bitis arietans). Proceedings of the Royal Society B: Biological Sciences, 2015; 282 (1821)
- Nilson G, Andrén C, Ioannidis Y, Dimaki M. 1999. Ecology and conservation of the Milos viper, Macrovipera schweizeri (Werner, 1935). Amphibia Reptilia;20(4): 355-75
- Phelps T. 2010. Old world vipers. A natural history of the Azemiopinae and Viperinae. Frankfurt am Main: Edition Chimaira.
- Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology, 13(1), 93.
- Shi J, Wang G, Chen X, Fang Y, Ding L, Huang S, Hou M, Liu J, Li P. 2017. A new moth-preying alpine pit viper species from Qinghai-Tibetan Plateau (Viperidae, Crotalinae) Amphibia-Reptilia, 38 (4): 517 – 532
- Shine R, Branch WR, Harlow PS, Webb JK. 1998. Reproductive Biology and Food Habits of Horned Adders, Bitis caudalis (Viperidae), from Southern Africa. Copeia (2): 391-401.
- Sochurek E. 1979. Die Schlangen Nordafrikas. Mitt. Zool. Ges. Braunau 3 (8/9): 219-226
- Spawls S, Howell K, Drewes R, Ashe J. 2004. A Field Guide to the Reptiles of East Africa. A & C Black Publishers Ltd., London. 543 pp.
- Wagner P, Wilms TM. 2010. A crowned devil: new species of Cerastes Laurenti, 1768 (Ophidia, Viperidae) from Tunisia, with two nomenclatural comments. Bonn Zool. Bull. 57 (2): 297–306
- Wallach V, Williams KL, Boundy J. 2014. Snakes of the World: A Catalogue of Living and Extinct Species. [type catalogue] Taylor and Francis, CRC Press, 1237 pp.
- Webber MM, Jezkova T, Rodríguez-Robles JA. 2016. Feeding Ecology of Sidewinder Rattlesnakes, Crotalus cerastes (Viperidae). Herpetologica: December 2016, Vol. 72, No. 4, pp. 324-330.
- Werner YL, Sivan N, Kushnir V, Motro U. 1999. A statistical approach to variation in Cerastes (Ophidia: Viperidae) with the description of two endemic subspecies, In U. Joger. (ed.): Phylogeny and Systematics of the Viperidae. Kaupia (Darmstadt) (8): 83-97
To cite this page:
Martínez del Mármol (2020): The phenotypic variability of the Genus Bitis Gray 1842, with remarks in its resemblance to other vipers. In: Martínez, G., León, R., Jiménez-Robles, O., González De la Vega, J. P., Gabari, V., Rebollo, B., Sánchez-Tójar, A., Fernández-Cardenete, J. R., Gállego, J. (Eds.). www.moroccoherps. com. Amphibians and Reptiles of Morocco and Western Sahara.
To cite www.morocoherps.com en as a whole:
Martínez, G., León, R., Jiménez-Robles, O., González De la Vega, J.P., Gabari, V., Rebollo, B., Sánchez-Tójar, A., Fernández-Cardenete, J.R., Gállego, J. (Eds.). Moroccoherps. Amphibians and Reptiles of Morocco and Western Sahara.
Available from www.moroccoherps.com.

