Iberian False Smooth Snake
Macroprotodon brevis (Günther, 1862)
By Gabriel Martínez del Mármol & Vincenzo Rizzo
Updated: 11/12/2020
Taxonomy: Serpentes | Colubridae | Macroprotodon | Macroprotodon brevis

 M. b. ibericus
M. b. ibericus
 M. b. brevis
M. b. brevis
 M. brevis “textilis”
M. brevis “textilis”
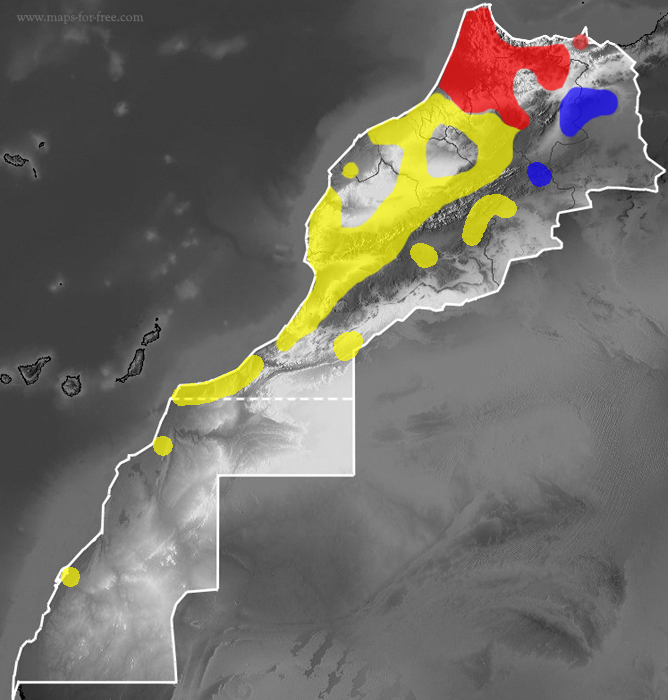
Tentative map of the distribution of Macroprotodon brevis in Morocco
1) Phylogenetic frame
Molecular studies have shown that Macroprotodon is the sister taxon of Bamanophis dorri (Lataste, 1888) (Nagy et al., 2003; Pyron et al., 2013), and show that Macroprotodon brevis is paraphyletic with the specimens morphologically identified as M. cucullatus textilis from eastern Morocco (Carranza et al., 2004, Vasconcelos and Harris, 2006; Faraone et al., 2020).
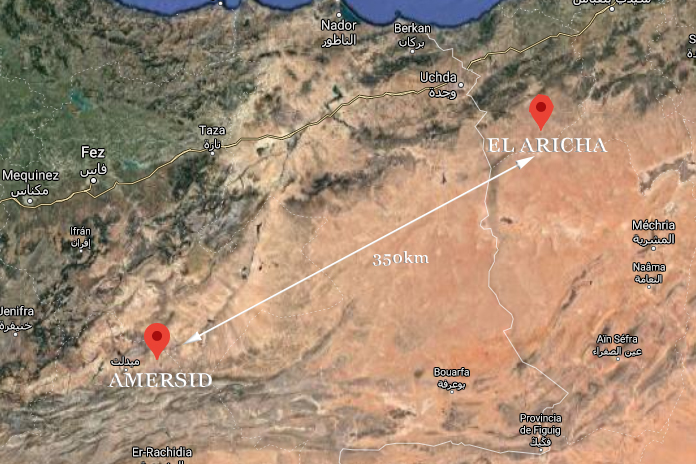
In the description made by Duméril & Bibron (1854), the type locality for M. textilis is vague: “du désert de l´ouest de l ´ Algerie” (=a desert in western Argelia), and for Doumergue (1901) they were referring to the Aricha region. These populations are approximately 350 km away from the specimens morphologically identified as M. cucullatus textilis from the plains of eastern Morocco (Amersid) that were genetically analyzed and which form a third lineage close to M. brevis ibericus and M. brevis brevis (Carranza et al., 2004).
On the one hand, regarding the morphological features, these do not seem completely reliable considering that it had also been identified as M. c. textilis a sample of Tizi n Tichka that finally appears grouped in the clade of M. brevis brevis (Carranza et al., 2004) or the coastal populations south of the Oued Draa that had been historically identified as M. cucullatus (Bons & Geniez, 1996) or M. cucullatus textilis (Wade 2001), and it has been probed that they belong to the brevis brevis clade (de Pous & Martínez del Mármol in Martínez del Mármol et al., 2019), as well as another specimen from Tunisia classified as M. c. textilis and that has been genetically included in the same clade as the rest of M. cucullatus of Tunisia, Libya, Balearic Islands or northeast Argelia (Carranza et al., 2004).
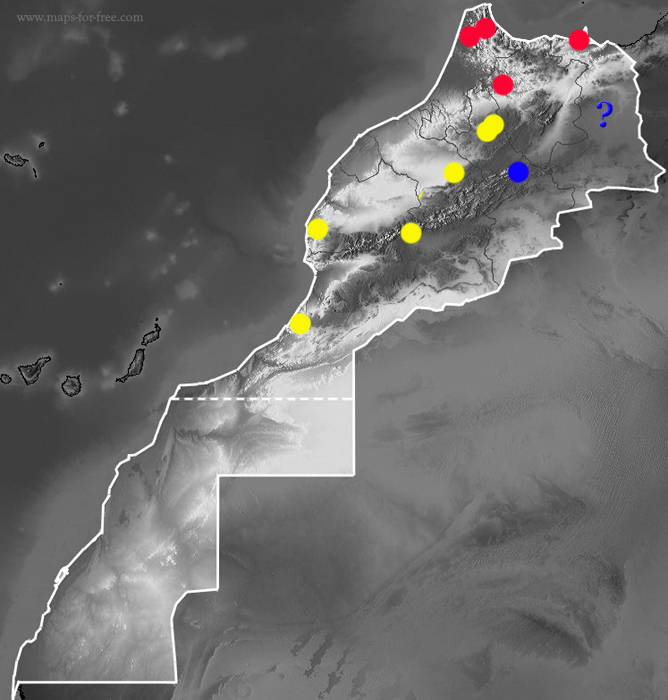
In addition, there are still many questions to answer, especially in the southern limits of Macroprotodon abubakeri and the possible contact areas with the lineage textilis and contact areas between the “textilis” and the eastern High Atlas populations of M. brevis. It would be essential to contrast the study of Carranza et al. (2004) with samples from the populations of arid zones south of the High Atlas (e.g. Icht, Assa, Agdz…), the plains of eastern Morocco (eg. Jerada, Taourirt…) to try to further clarify the distribution of this genus in North Africa, as Pleguezuelos & Vasconcelos (2015) point out the possibilities of El Aricha y other areas of western Argelia after being genetically analyzed are diverse: that they belong to the brevis clade, such as the Amersid specimens analyzed by Carranza and collaborators (2004), secondly that they belong to M. cucullatus s.s. which would expand the distribution of this species from Israel to western Argelia (or even eastern Morocco), or thirdly that they are a clade different from the rest and textilis can be considered a own species.
Geniez (2015) based exclusively on morphology and the absence of significant natural barriers that suggests that it is the same lineage, since many other species of reptiles present in western Algeria have expanded through the arid steppes to eastern Morocco (e.g. Ophisops occidentalis, Psammodromus blanci, Trogonophis wiegmanni wiegmanni, Eryx jaculus) seems to follow the first of the theories mentioned in the previous paragraph and thus considers that M. cucullatus textilis should be included within M. brevis, which was also reflected in later works (Martínez del Mármol et al., 2019). Thus, for these authors in Morocco Macroprotodon brevis would have 3 subspecies: brevis, ibericus and textilis. In the present work we will also consider this classification only provisionally, since if it is confirmed that the populations of western Algeria belong to the same lineage as those of Amersid, we understand that the appropriate thing would be to consider that the name Macroprotodon textilis prevails for the species, since Lycognathus textilis Duméril & Bibron 1854 is older than Coronella brevis Günther 1862, as indicated by Carranza et al., (2004).
The absence of more samples in the molecular studies on the genus Macroprotodon, specifically from eastern Morocco and western Algeria, and the low reliability of the morphological data, mean that any classification should be taken with caution until future molecular analyzes can help to understand more precisely the actual distribution of the different species of this genus in North Africa.
2) Description
Macroprotodon brevis is a small species of snake, normally not exceeding 50 cm in total lenght; although there are records of individuals over 60 cm (Schleich et. al., 1998; Speybroeck et. al., 2016; Martínez del Mármol et. al., 2019).

It has a head little differentiated from the body and characteristically depressed, with a short and wide snout (adaptations to its fossorial life). Their eyes are very small (another adaptation to their fossorial ecology) with a round pupil. It usually has a dark mark that goes from the nostrils to the last supralabial in contact with the eye, or even to the last supralabial scale passing through the eye. Most of the specimens have a “U” or “V” shaped mark on the top of the head, which is negligible or almost imperceptible in partially melanocephalic specimens (with a large amount of black pigmentation on the head) or in individuals with little contrast.
All subspecies (except populations of M. b. textilis and some specimens of M. b. brevis that have the hood much less contrasted and divided into three parts) present a dark area (more or less contrasted depending on the individual and / or of populations) as a hood (hence the common name “hooded snake”) that occupies the back of the head and neck (Schleich et. al., 1998; Wade, 2008; Speybroek et al., 2016; Martínez del Mármol et al., 2019).

Its body, although robust, is relatively elongated and covered with smooth scales. Its tail is very short in proportion to the size of the body.
The background color of the body is quite variable, being beige, cream, light gray, light brown, reddish brown, sand or olive (Schleich et al., 1996; Geniez et. al., 2015; Speybroeck et. al., 2016). Its body is covered by rows of small dark spots (which can be more or less contrasted depending on the individual and / or population) that can give it a tessellated appearance; in some cases, these spots may come together, forming fine rows; and in other cases, these spots are almost imperceptible. There are some individuals that have a pattern of large dark spots that contrast with the background, but they are a small minority. There can be a great variety of designs and colors even within the same population (Martínez del Mármol et al., 2019).
The belly is also of variable coloration, being able to be white, pink, yellow, beige, cream or reddish. It can also be checkered (with “square” black spots alternated with the background color) or completely uniform (Schleich et al., 1996; Geniez et al., 2015; Speybroeck et al., 2016).
Juveniles are a miniature copy of adults, although they tend to have more contrasting designs.
There are some morphological characteristics that can differentiate the different subspecies:
- Macroprotodon brevis ibericus: complete hood except in melanocephalic specimens. Its dorsal pattern is usually made up of very small spots separated from each other in rows, sometimes these spots are fused to form fine rows and, in other individuals, these spots can be negligible. Checkered belly. Normally 21 rows of dorsal scales in the middle of the body (Geniez 2015, Martínez del Mármol et al., 2019) although Pleguezuelos (1998) indicates the presence of specimens with 19, 20, 22 and 23 dorsal rows in specimens from the Iberian Peninsula.
- Macroprotodon brevis brevis: most populations have the entire hood, but southern populations close to the distributional border with M. textilis usually present (like the previous subspecies) the collar divided into three little contrasting parts (this does not happen with individuals with melanocephaly). Its pattern is variable, being able to have a tessellated pattern (like that of M. b. textilis), have smaller and more separated spots, have a clear reticulum in the separation of the spots or, in some cases, have large well-contrasted spots with the base. Its belly is usually checkered (except for the populations of the distribution limit with M. b. textilis, which are indistinguishable from it only with morphology (from Pous and Martínez del Mármol in Martínez del Mármol et al., 2019). It is 19, 21 o 23 rows of mid-body dorsal scales (Geniez, 2015; Martínez del Mármol et. al., 2019).
- Macroprotodon brevis textilis: it usually presents the hood divided into three parts (often this is not very noticeable in poorly contrasted individuals; in melanocephalic specimens there is no divided hood as it has the entire upper part of its head). Its body is very mottled, with medium spots; the arrangement of these spots gives it a very characteristic tessellated pattern (hence the scientific name textilis). Its belly is usually whitish, yellowish or light pink, the coloration is uniform and it is never checkered. It has 19 rows of dorsal scales in the middle of the body (Geniez, 2015; Martínez del Mármol et al., 2019).

Similar species
In Morocco, Macroprotodon brevis can be confused mainly with three snakes: its relative, M. abubakeri (Abubaker’s False Smooth Snake), with Coronella girondica (Southern Smooth Snake) and with Telescopus tripolitanus (North African Cat Snake).
The Western False Smooth Snake can be differentiated from Macroprotodon abubakeri in its pattern, that of the latter is usually of small spots located in two rows in the vertebral area (this pattern is absent in M. b. textilis, although possible in M. b. ibericus) with a checkered belly (M. b. textilis has a smooth belly, but M. b. ibericus also has a checkered belly) and the stria that crosses the eye is usually connected to the hood (this does not usually happen in M . brevis); but the most reliable way to achieve a correct identification is by its distribution, that of Abubaker is located in the extreme northeast of Morocco (very close to the Algerian border); west of this point is M. b. ibericus and to the south of this we find M. b. textileis (Wade, 2001; Martínez del Mármol et. al., 2019). However, it is foreseeable that there will be contact areas between both species in both Morocco and Algeria.
Coronella girondica usually has a pattern with horizontal bars all over the body, it always has a checkered belly (while M. b. textilis has it uniform) and it never has a hood.
In some areas of its distribution, melanocephalic specimens could inhabit in sympatry with Telescopus tripolitanus (e.g. south of Assa in Kane et al., 2019; Agdz, unpublished data). The design of the body is different but the easiest thing to distinguish them is their pupil, round in Macroprotodon and vertical in Telescopus.

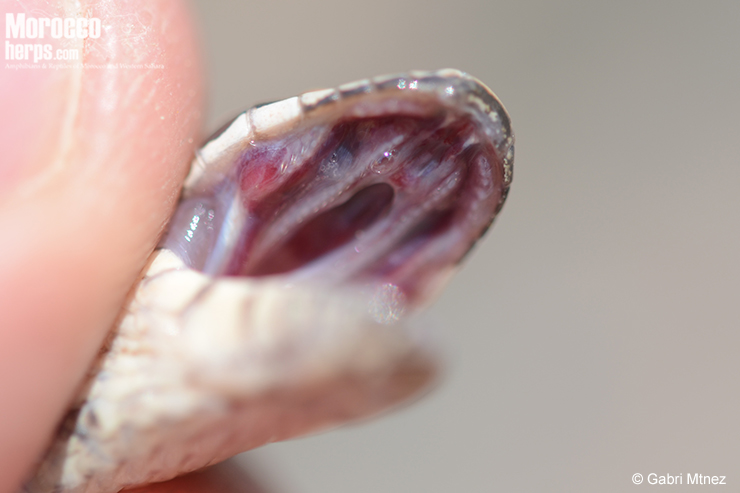
Dentition
It is an opisthglyph species, presenting fixed grooved fangs connected to Duvernoy’s glands in the rear of the upper jaw. Their venom is very mild and helps them succumb to their prey, but it is totally harmless to humans (apart from the low potency of the poison, its arrangement at the back of the jaw and its small size make it extremely unlikely that a human being poisoned).
3) Ecology and habits
The Iberian cogulla snake is an eminently terrestrial snake that develops its activity mainly in galleries and under stones, coming to the surface mainly at twilight and the first hours of the night, although in Andalusia it has also been seen active during the first hours of in the morning or at another daytime hour on cloudy days (González de la Vega, 1988).
In mountainous areas of Morocco it must have a period of inactivity in the coldest months, while in areas of mild winters such as Sidi-Ifni, Tantan or Tarfaya we have observed active specimens from March to December, so it may be active during practically all the year as also indicated by Schleich and collaborators (1996) for the genus Macroprotodon in general.
The males of this species, despite their African origin, have summer spermatogenesis, this allows them to have sperm from immediately after winter torpor, so copulations can begin very early in the spring. The mating period lasts until June. This extended copulation period is probably selective in a species with low vagility (J. M. Pleguezuelos, pers. comm.). It is assumed that there are few male-female contacts, so they have to take advantage when this happens and despite being a very elusive species, copulations can be seen even on the surface in broad daylight in crowded areas (Toledano et al., 2020 ). The male bites the female during copulation, which lays 2-6 eggs between one and two months later. It is believed to have a low metabolism and reproduce every two years. Neonates are seen in the wild from mid-August to the end of September (González de la Vega, 1988; Pleguezuelos, 2015).

It can feed on invertebrates, small mammals or small bird chickens, but it is above all an ophidian specialist in feeding on other reptiles. Small lizards, agamids, snakes (including their own species) and very frequently amphisbenids make up the majority of their diet. Unlike other colubrids that hunt for active foraging, the Western False Smooth Snake seems to hunt by ambushing its prey under stones and waiting for them to appear, at which point it bites them and inoculates the venom to favor swallowing prey that is sometimes very large in relation to the predator (possibly the largest prey-predator size ratio of all Mediterranean snakes; Pleguezuelos, 2015).

Cases of predation by the Montpellier snake (Malpolon monspessulanus), the mongoose (Herpestes ichneumon) and the red kite (Milvus milvus) have been cited (Pleguezuelos, 2015). Due to its habit of living under small stones, it is very possible that it is also preyed upon by wild boars (Sus scrofa) (Mateo et al., 2003; Pleguezuelos & Feriche, 2003). The Western False Smooth Snake, in case of being surprised by a potential predator, usually chooses to either imitate a viperid by launching attacks and adopting a dock position, or by hiding its head under its body. Its small size and the position of the teeth that inoculate venom in the back of the upper jaw mean that there are no known cases of poisoning in humans (Schleich et al., 1996)
4) Distribution, habitat and abundance
The Western False Smooth Snake is found mainly in the southern half of the Iberian Peninsula and in North Africa, pending genetically confirmation of its presence in Eastern Morocco and Algeria (Geniez et al., 2015; Martínez del Mármol et al., 2019). The rest of Macroprotodon populations formerly considered as M. (c). textilis are actually M. cucullatus s.s. (Carranza et al., 2004), with the exception of the populations of Lampedusa and part of Tunisia, which probably constitute a species not yet described (Faraone et al., 2020).
It is distributed practically all over Morocco except in a small area in the extreme northeast of the country (near the border with Algeria, where it is replaced by M. abubakeri) and in the arid areas south of the High Atlas, where it only manages to penetrate through oases or favored by coastal climatology (Geniez et al., 2015; Martínez del Mármol et al., 2019).
By subspecies:
- M. b. brevis is a Moroccan endemism with a wide distribution in the study area, except in the north where it is replaced by M. brevis ibericus (northwest), M. abubakeri (northeast) and to the east where M. b. textilis.
- M. b. ibericus can be found along the Rif Mountain Range up to Cap des Trois Fourches (at the eastern end), in Jbel Bou Iblane and in the Tingitana Peninsula, reaching Moulay Bousselham to the south (Martínez del Mármol et. al., 2019).
- M. b. textilis: its distribution in Morocco in the arid zones east of the Atlas Mountains is presumed according to morphological analysis (Wade, 2001), although only a lineage other than M. brevis brevis has been confirmed in Amersid (Carranza et al., 2004 ) that some authors attribute to the textilis subspecies (Geniez et al., 2015; Martínez del Mármol et al., 2019).
This snake occupies a wide spectrum of habitats and seems more dependent on the food available than on the type of habitat, although in general it seems more frequent in wooded areas, meadows or coastal areas with a soft enough soil to be able to carry out its fossorial life. Some examples of suitable habitats for this colubrid are coastal maquis (often with palm hearts, Chamaerops humilis), dunes and other sandy areas, open forests, steppes, ruins, orchards … (Schleich et al., 1996; Speybroeck et al., 2016; Martínez del Mármol et al., 2019) and we have also found it in areas highly altered by humans such as crops dedicated to intensive agriculture in the Fes region (Sánchez Vialas A., Jiménez Robles O., Martínez del Mármol G, unpublished data). Indeed, the Western False Smooth Snake is one of the reptiles that can most frequently be found in dumps, trash areas, edges of crops, lots and other places that have been greatly altered by humans.

It occupies an altitudinal range from sea level to 2080 m (Martínez del Mármol et al., 2019), although a single specimen has been cited above that altitude, specifically at 2,500 m in the High Atlas (Bons & Geniez, 1996 ; Schleich et al., 1996). This requires confirmation and that it is not a confusion with Coronella girondica, a typical species at that altitude in Morocco.
Macroprotodon brevis is a species that can be found easily if it is searched in suitable places, apparently reaching high densities. It is declared as “NT” (near threatened) and as “declining population” by the IUCN Red List of Threatened Species (Joger et al., 2009).
Its main threats are accidental fall traps (where a huge number of animals die each year from drowning, starvation or dehydration because they cannot get out of them) and the roads where large numbers of run-over individuals die.

Ackowledgements
To Juan Manuel Pleguezuelos and Juan Pablo González de la Vega for their contributions on the taxonomy and biology of the genus Macroprotodon. To Juan Pablo González de la Vega and Jesús Toledano Portal for their photographs.
Bibliography
- Bons J & Geniez P. 1996. Anfibios y Reptiles de Marruecos (Incluido Sahara Occidentales). Atlas Biogeográfico. Asociación Herpetológica Española. Barcelona. 319 pp.
- Carranza S, Arnold EN, Wade E, Fahd S. 2004. Phylogeography of the false smooth snakes, Macroprotodon (Serpentes, Colubridae): mitochondrial DNA sequences show European populations arrived recently from Northwest Africa. Molecular Phylogenetics and Evolution, 33: 523-532
- Doumergue F. 1901. Essai sur la faune herpétologique de VOranie. Fouqué éd., Oran 404 pp. Extract from Bulletin de SocieÅLteÅLgeÅLographie et Archeeologie d_Oran 19–21 (1889–1900).
- Duméril AMC, Bibron G & Duméril AHA., 1854. Erpétologie générale ou histoire naturelle complète des reptiles. Tome septième. Deuxième partie, comprenant l’histoire des serpents venimeux. Paris, Librairie Encyclopédique de Roret: i-xii + 781-1536
- Faraone FP, Melfi R, Di Nicola MR, Giacalone G. 2020. The genetic identity of the only Italian population of the genus Macroprotodon Guichenot, 1850 on the island of Lampedusa, Sicily. Vertebrate Zoology 70 (2): 235-240.
- Geniez P. 2015. Serpents d’Europe, d’Afrique du Nord et du Moyen-Orient. Delachaux et Niestlé, Neuchâtel, 380 pp.
- González de la Vega JP. 1988. Anfibios y Reptiles de la provincia de Huelva. Ertisa, Huelva.
- Kane D, Goodwin S, Verspui GJ, Tump A, Martínez del Mármol Marín G. 2019. Reptile diversity of southern Morocco: range extensions and the role of the Djebel Ouarkziz as a biogeographical barrier. Herpetology Notes 12: 787-793
- Martínez del Mármol G, Harris DJ, Geniez P, de Pous P, Salvi D. 2019. Amphibians and Reptiles of Morocco. Frankfurt, Germany, Edition Chimaira. 478 pp.
- Mateo JA, Pleguezuelos JM, Fahd S, Geniez P, Martínez-Medina FJ. 2003. Los anfibios, los reptiles y el estrecho de Gibraltar. Un ensayo sobre la herpetofauna de Ceuta y su entorno. Instituto de Estudios Ceutíes. 388 pp.
- Nagy ZT, Lawson R, Joger U & Wink M. 2003. Molecular sustematics of racers, whipsnakes and relatives (Reptilia: Colubridae) using mitochondrial and nuclear markers. Zool. Sust. Evol. Research, 42: 223-233.
- Pleguezuelos JM. 1998. Macroprotodon cucullatus (Geoffroy de Saint-Hilaire, 1827). En: Fauna Ibérica, vol. 10. M.A. Ramos et al. (Eds.). Museo Nacional de Ciencias Naturales. C.S.I.C. Madrid: 428-439.
- Pleguezuelos JM & Feriche M. 2003. Anfibios y reptiles (Los libros de la estrella, 18). Diputación de Granada, 185 pp.
- Pleguezuelos JM. 2015. Culebra de cogulla occidental – Macroprotodon brevis. En: Enciclopedia Virtual de los Vertebrados Españoles. Salvador, A., Marco, A. (Eds.). Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/
- Pleguezuelos, J. M. & Vasconcelos, R. 2015. Culebra de cogulla argelina – Macroprotodon cucullatus. En: Enciclopedia Virtual de los Vertebrados Españoles. Salvador, A., Marco, A. (Eds.). Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/
- Pyron A, Burbrink FT & Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13: 93.
- Schleich HH, Kästle W & Kabisch K. 1996.Amphibians and Reptiles of North Africa. Koeltz Sci. Books, Koenigstein. 630 pp.
- Speybroeck J, Beukema W, Bok B, Van der Voort J. 2016. Field Guide to the Amphibians and Reptiles of Britain and Europe. Bloomsbury. 432 pp.
- Toledano Portal J, León Vigara R. & Martínez del Mármol, G. 2020. Nuevos datos sobre la cópula de la culebra de cogulla occidental Macroprotodon brevis. Boletín de la Asociación Herpetológica Española, 31 (1): 56-58.
- Vasconcelos R & Harris DJ. 2006. Phylogeography of Macroprotodon: mtDNA sequences from Portugal confirm European populations arrived recently from NW Africa. Herpetozoa, 19(1/2): 77-81.
- Wade, E. (2001). Review of the False Smooth snake genus Macroprotodon (Serpentes, Colubridae) in Algeria with a description of a new species. Nat. Hist. Mus. Lond. (Zool.), 67 (1): 85-107.
To cite this page:
Martínez del Mármol G & Rizzo V. 2020. Macroprotodon brevis (Günther, 1862). In: Martínez, G., León, R., Jiménez-Robles, O., González De la Vega, J. P., Gabari, V., Rebollo, B., Sánchez-Tójar, A., Fernández-Cardenete, J. R., Gállego, J. (Eds.). Moroccoherps. Amphibians and Reptiles of Morocco.
Available from www.moroccoherps.com/en/ficha/Macroprotodon_brevis/ Version 11/12/2020.
To cite www.morocoherps.com as a whole:
Martínez, G., León, R., Jiménez-Robles, O., González De la Vega, J.P., Gabari, V., Rebollo, B., Sánchez-Tójar, A., Fernández-Cardenete, J.R., Gállego, J. (Eds.). Moroccoherps. Amphibians and Reptiles of Morocco and Western Sahara. Available from www.moroccoherps.com

